2020. 2. 15. 03:32ㆍ카테고리 없음
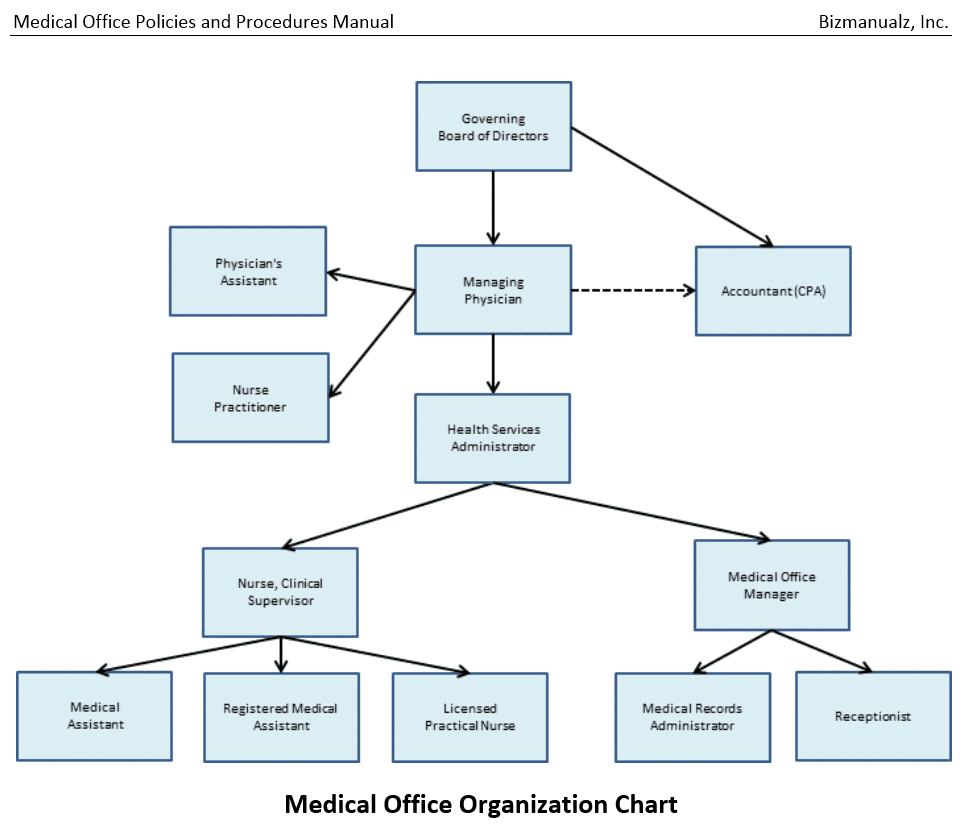
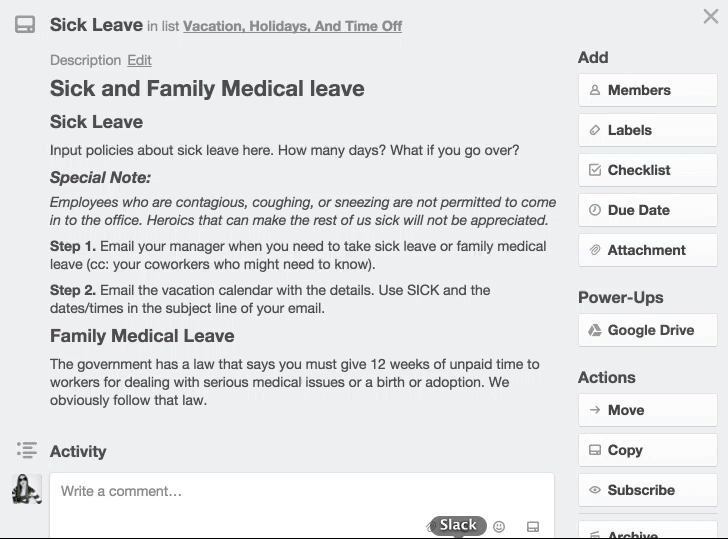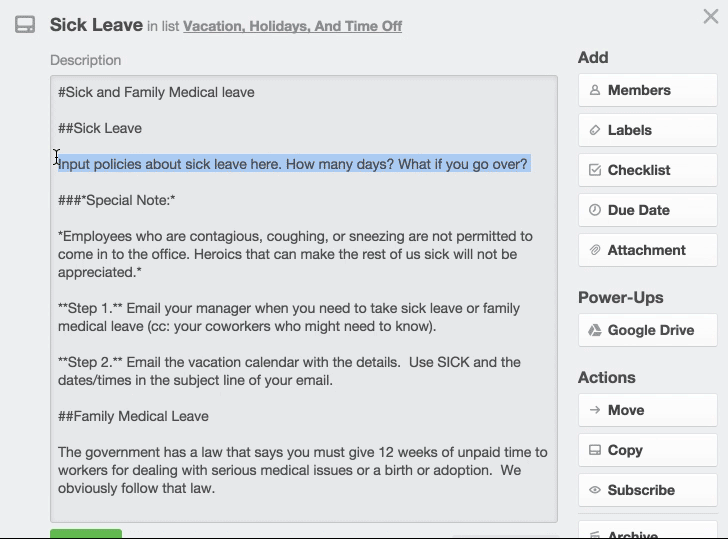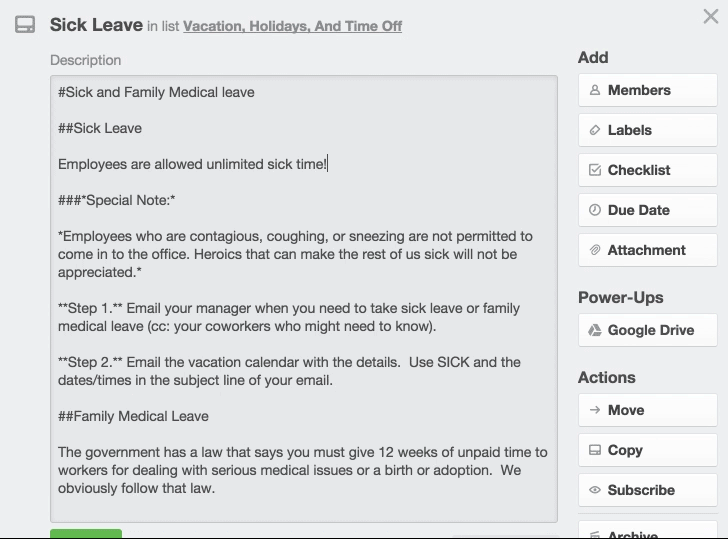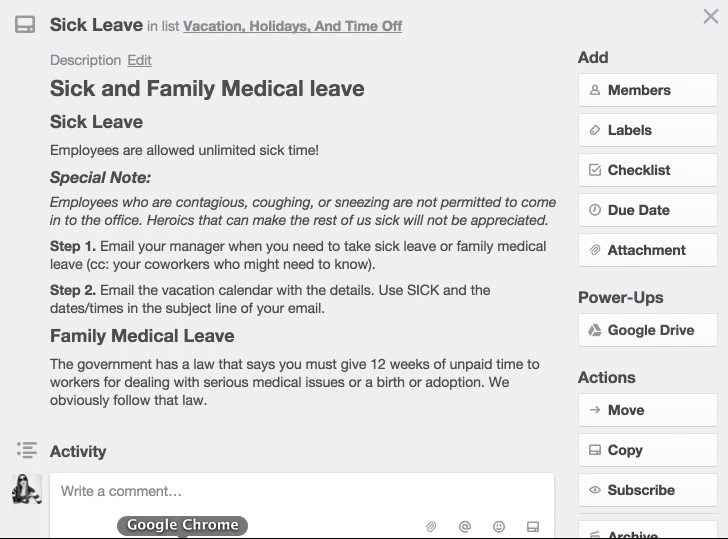

Medical Office Employee Training Manual
. Medical Device Quality Systems Manual with 820 and QSR Audit Checklist 978-1-935131-06-9 US FDA Title 21 CFR Parts 21 CFR Part 820 - Quality Systems Regulations QSR Audit Check List Medical Device Quality System Manual Quantity 1 - 99 100 - 249 250 - 499 500 - 999 1000+ Price $15.90 $15.50 $13.90 $11.90 $9.90 Quantity Other GMP Products that you may be interested in: You have the books, now get the training! GMP Publications Special - $4,995.00 - All Inclusive 2 day bootcamp seminar - Includes Course Binders, Handbooks, and Certificates for up to 20. Attendees, Travel, Hotels and Expenses - Conducted by The Auditing Group. For more details, call 856-810-7331 -.Additional attendee binders (over 20) will be charged $25.00 USD each.